Ep. 9: Formation of the Heaviest Elements
Have you ever wondered how all the chemical elements are made?
Then join me as we are lifting all the star dust secrets to understand the cosmic origin of the chemical elements.
We talked a lot about the lighter elements that are made in fusion processes
in the cores of stars but what about all the other elements
from the bottom half of the periodic table?
We haven't really talked about those yet -- Let's do that!
What we need are so-called seed nuclei. We have an iron nucleus, here, and if we are
in a situation where there is a strong neutron flux available (we'll talk about
where that happens in second) then if we have little neutrons here and this seed
nucleus is getting bombarded with these neutrons then it's going to swell and
turn into a much larger nucleus that is radioactive and neutron-rich and it's an
isotope that has lots of neutrons in it. What's happening then, because it was
radioactive, it doesn't like to stay in this way, it will what we call
beta-decay which is just a fancy word for saying that all these neutrons here are
being converted, or good fraction of them, into protons, and so we end up with a
stable element that's much larger than this original one,
the iron. Or it could also have been a carbon atom. This is the basic idea how all the
other heavy elements are made. An example will be barium here or uranium. Uranium-238
is technically not a stable element but it's half-life is 4.7
billion years so for us humans that's pretty stable but on cosmic
timescales it is not. But it if we want to consider it as stable it would be the
heaviest element that we have on Earth that's long-lived. They're all
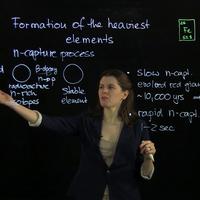
made by this so-called neutron-capture process -- neutron-capture process. Now
there are a few details that we should consider, mostly that there are
actually two different ways where this neutron-capture
process can happen. One is in a slow way -- slow neutron-capture -- and
the other way is rapid. And that refers to how fast and over
what timescale this neutron bombardment is occurring. In the case of the slow
neutron-capture, the timescale is about 10,000 years, and what happens is that in
evolved red giant stars -- evolve red giants -- in the inner layers in
some of the shell layers where the nuclear fusion is going on, and there
are secondary nucleosynthesis fusion processes operating, and as a result of
that free neutrons are produced. They provide a steady flux of neutrons
that then get essentially shot onto these seed nuclei, and so over the
timescale of something like 10,000 years, heavy elements are successively
build up. A neutron is added, it turns into radioactive isotope, it decays and then
you have a steady one. You add another neutron, it will decay again, and so you
kind of it build up, one by one by one, all the way up to lead. It's the heaviest
stable-stable element, if you take away thorium and uranium because they *are*
radioactive, as I just mentioned. Now in the case of the rapid neutron-capture
that really requires much more energetic and extreme conditions, and what recent
research has shown is that rapid neutron flux only operates in two locations. One
is perhaps in supernovae. When the iron core collapses at the end of
star's life, it actually implodes and forms a neutron star. There's a
really dense neutron star in the middle -- that's a contact remnant left over after
the supernova -- and in the process of making this neutron star, there are of
course lots of neutrons floating around. They can provide this kind of flux,
operating on a 1 to 2 second timescale. So a huge neutron bombardment
within a few seconds and that can lead to a very, very fast buildup of
giant radioactive nuclei here that then decay. So if you have enough seed
nuclei and all of them would make "whoom" [become huge] and then slowly decay back to the
different elements that make up the entire bottom of the periodic table.
Another option is merging neutron stars. If you take two of these neutron
stars, and you have them in a binary system where they orbit each other, and
if this system eventually, or the two stars in the system, eventually coalesce
and merge, then you also have some kind of firework of neutrons, and that can
also have this rapid neutron-capture going on. We have to add here, so
either in supernovae or the proto-neutron star if you have like that
or in neutron star mergers. That are all the options and what
we now want to really figure out is how can we put all this theory to the test,
right, how can we observe this? That's when our old stars come
back into play. Imagine that in the very beginning of the universe, when
the first stars emerged and maybe the second generation of stars, so not too
much of all the heavy elements was present at that time. Let's
say, you have a neutron star merger go off at this very early time, the rapid
neutron-capture process will occur, and all these new heavy elements gets spilled
into the surrounding, and then you form a next-generation star from this enriched
material. And because the universe was not too much enriched in all the
other elements, we have this opportunity to observe a clean nucleosynthesis
process of this r-process (it's sometimes abbreviated' rapid' with r) so 'r-process'.
And actually, this here is s-process,
you could have guessed that. So at the earliest times, it is possible
to observe the signature of the r-process, a clean signature, as well as the s-process.
That is not possible anymore today. The universe has experienced
13 billion years of chemical evolution, so it's a pretty messy place
out there. If one more event goes off, that signature just gets
diluted into whatever else is out there. But at the earliest times, we have this
chance to find these clean signatures.